BRAND NEW: VivaChek Rapid Gold Pro SARS-CoV-2-Ag rapid test CE1434 (pack of 1)
VivaChek Rapid Gold Pro lay test for reliable detection of a COVID-19 infection
Very good overall sensitivity even with a low viral load | Evaluated by the Charité Berlin | CE certified (CE1434)
If you need larger quantities than our online scale, please contact our customer service directly!
*Of course, we would be happy to send you the Charité test report upon request. We ask for your understanding that we do not make this freely accessible here in order to prevent misuse.
Product Details
- Layer test for nasal use
- Evaluated by the Charité Berlin according to the criteria of the PEI
- extremely high sensitivity with low viral load
- CE certified CE1434
- Available in packs of 1, 5 and 25
- prefilled buffer solution
- Tube holder included in package
Suitable for:
- Nursery & Schools
- Municipalities & Municipalities
- Healthcare
- Company
- Private use at home
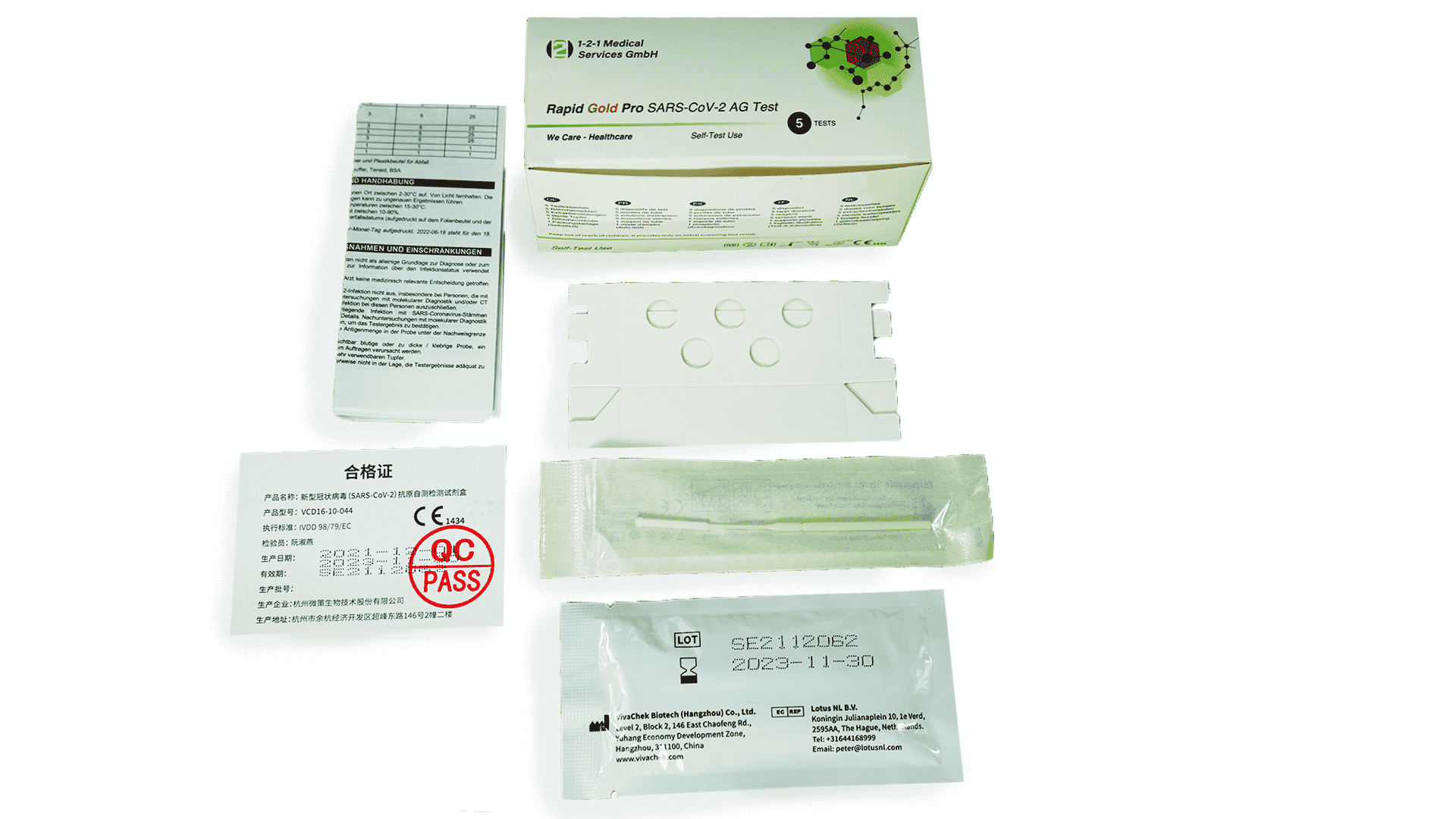
Scope of delivery (test components included)
- 1x test cassette
- 1x extraction buffer
- 1x leaflet
- 1x Sterile Swab
- 1x tube holder
Instructions for use of the Rapid Gold Pro SARS-CoV-2-Ag rapid test
The Rapid Gold Pro SARS-CoV-2-Ag rapid test is intended for the rapid, qualitative detection of the nucleocapsid protein antigen of SARS-CoV-2 in humans. The test is for in vitro diagnostic use only. It is intended for self-testing and only provides an initial screening test result. More specific alternative diagnostic methods (molecular diagnostics and/or CT) should be performed to confirm SARS-CoV-2 infection. The decision on the diagnostic procedure should be made by the physician. This test is intended for home use with self-collected nasal swab samples in individuals 16 to 69 years of age. Sampling and testing of persons under the age of 16 and persons over the age of 69 should be carried out under the supervision of an adult. For people who cannot perform the test themselves, the test should be
be carried out by legal guardians, sick/restricted people (including color vision deficiency) should be supported with the test.
1) Sampling
Swab from the front of the nose (anterior nasal)
Wash your hands with soap and water or use a hand sanitizer. It is important to get as much secretion as possible. Open swab pack at stem end and remove swab. Do not touch the swab head. Insert the sterile swab into one nostril. Make sure the swab tip is fully inserted into the nostril (about 1.5 cm). Roll the swab along the lining of the nasal wall 5 times to ensure that both mucus and cells are collected. Repeat this process in the other nostril to ensure an adequate sample is collected from both nostrils (use the same swab).
2) Sample handling
Specimens should be tested as soon as possible after collection (we recommend testing within 5 minutes).
Test execution
Bring the test cassette and buffer solution to room temperature (15-30°C) before testing.
1. Open the extraction solution (in the sealed tube).
2. Collect the sample, see Sampling.
3. Insert the swab containing the collected sample into the extraction tube filled with buffer solution. Roll the swab 5 times while pressing the head against the bottom and side of the tube. Remove the swab while squeezing the sides of the tube to force the liquid out of the swab. Try to squeeze out as much liquid as possible.
4. Insert the tube tip.
5. Remove a test cassette from the sealed foil pouch and place it on a clean, flat surface.
6. Add 3 drops of the extracted sample to the sample well. Please avoid blistering when applying.
7. Read the test result after 15 minutes. Do not read the result after 20 minutes.
QUALITY CONTROL
The test includes internal procedural controls. A colored line appearing in the control area (C) is the internal procedural control. This procedural control line indicates that adequate flow has occurred and the functional integrity of the test device is maintained.
Sensitivity and Specificity of Rapid Gold Pro SARS-CoV-2-Ag Rapid Test (Clinical Performance)
Specificity >99.99% (460/460, 95%CI, 99.17%~100%)
Accuracy 99.83% (574/575, 95%CI, 99.02%~99.97%)
The University Medicine of the Charité Berlin has also carried out an evaluation of the Rapid Gold Pro rapid test. Here, the Charité orientated itself exactly to the defined criteria of the Paul-Ehrlich-Institut in relation to the Ct values. The evaluation resulted in the following values for an average virus concentration (ct<25>30) or a low virus concentration (ct>30):
- Ct>25<30 value: 94.8% (147/155)
- Ct>30 value: 93.7% (148/158)
- Overall sensitivity: 92.5%
Limit of Detection
The limit of detection (LoD) per inactivated virus culture is 75.5 TCID50/mL.
The detection limit for the Rapid Gold Pro SARS-CoV-2 Ag Rapid Test was determined using dilutions of an inactivated virus culture (heat-inactivated SARS-CoV-2 isolate USA WA1/2020, NR-52281). The starting material was supplied at a concentration of 1.51×106 TCID50/mL. Studies were designed to estimate the limit of detection (LoD) of the assay using anterior nasal mucosal swab specimens.
Downloads
- Instructions for Use
- Declaration of Conformity
- Manufacturer’s declaration Omicron proof
- CE Certificate
Notes on storage
- Store the test in a cool, dry place between 2-30°C. Keep away from light. Storage outside of the specified conditions may lead to inaccurate results.
- Do not freeze. Use the test at temperatures between 15-30°C.
- Use the test at a humidity between 10-90%.
- Do not use the test after the expiration date (printed on the foil pouch and box).
Note: All expiration dates are printed in year-month-day format. 2022-06-18 stands for the 18th
June 2022.
Manufacturer
VivaChek Biotech (Hangzhou) Co., Ltd.