WANTAI SARS-CoV-2 Ag Rapid Test (Colloidal Gold) AT1265/21
Lollipop test for self-use | Suitable for children from 0 years | CE certified | AT1265/21
This Covid-19 lay test can be carried out using 2 methods: (1) by taking saliva under the tongue (lollipop test) or (2) a swab in the front of the nose. This means that sampling is less invasive and deep penetration into the nasopharynx is no longer necessary. That is why this rapid test is very often used in children.
Product features
- Selectable sampling method: nasal or lollipop test
- Suitable for children from 0 years
- Individually wrapped
- Integrated buffer solution tube
- Exact dosing
- Sterile Swab
- Plastic bag for contaminated waste
- PEI listed (now EU Common List) under AT1265/21
- Evaluated according to the guidelines of the RKI and the Paul Ehrlich Institute
- CE 2854
- Easy to use: Fast and reliable test results in just 15 minutes
Suitable for:
- Nursery & Schools
- Municipalities & Municipalities
- Healthcare
- Company
- Private use at home
If you need larger quantities, please contact us personally.
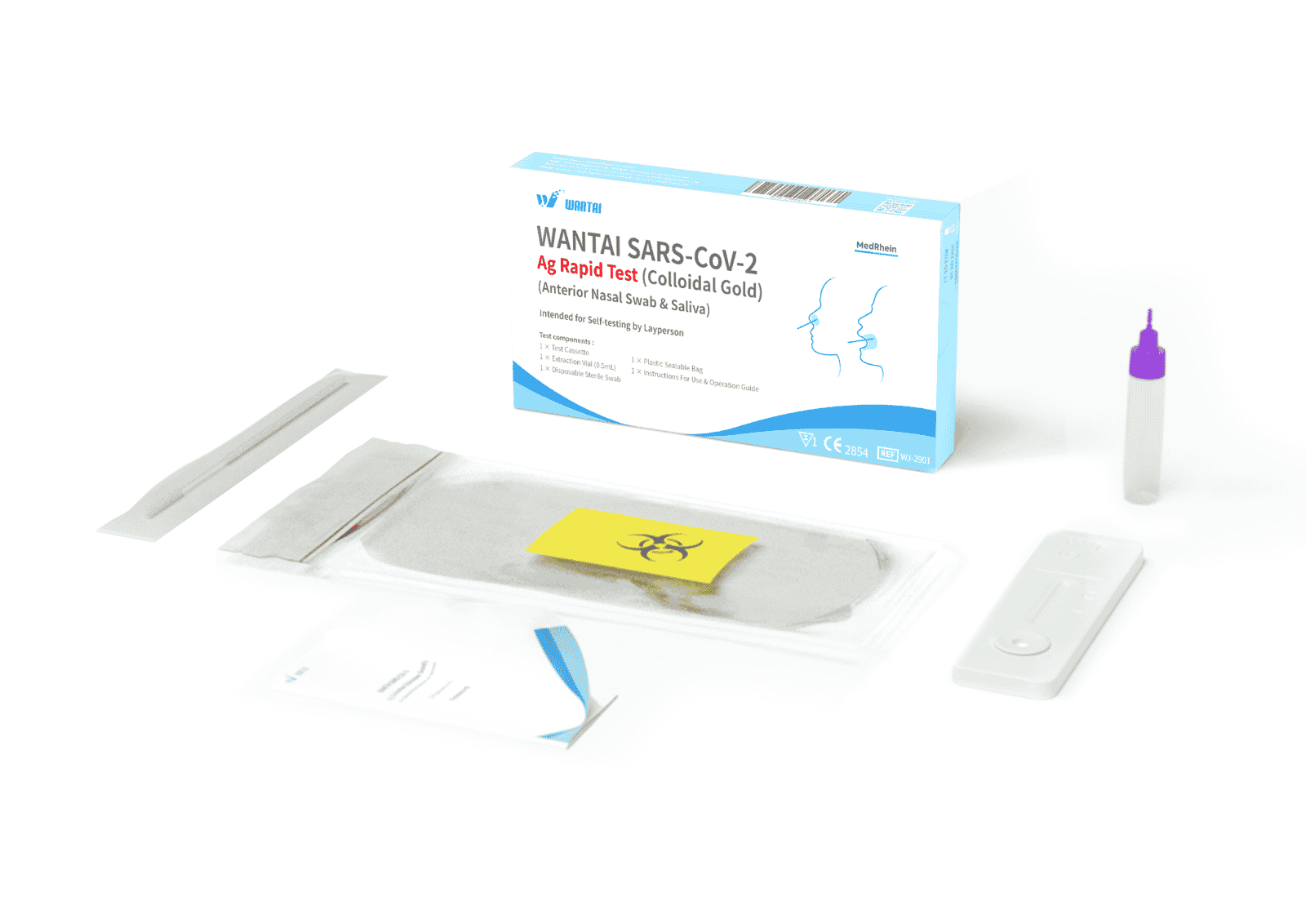
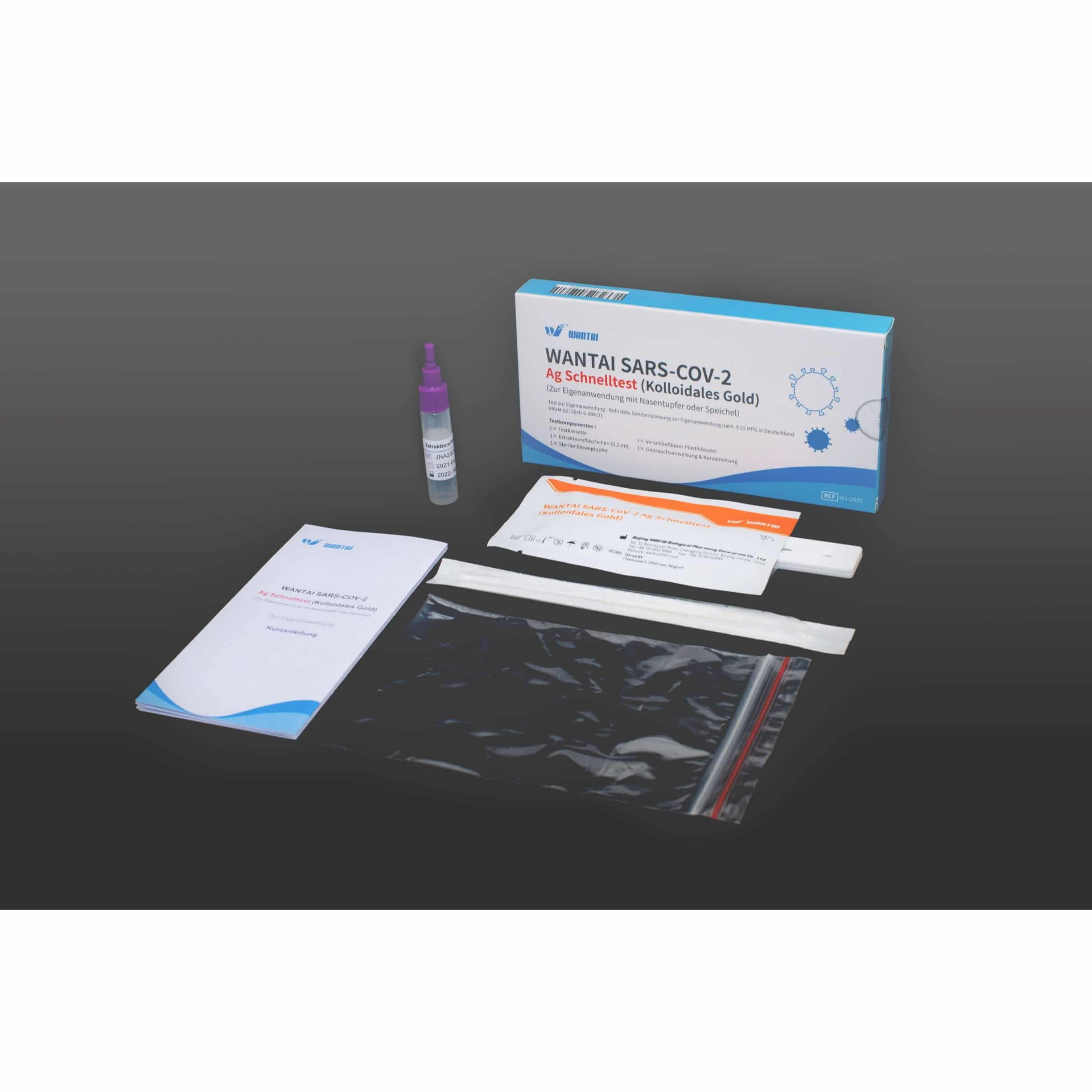
Scope of delivery (package contents)
- Test cassette x1
- Extraction Vial (0.5mL) x1
- Disposable sterile swab x1
- Resealable plastic bag x1
- Instructions for use & Quick Start Guide x1
Test cassette: The test cassettes are packed in foil pouches with desiccant. Each foil pouch contains 1 cassette. They are for single use only. Anti-SARS-CoV-2 antibody (anti-N protein) is coated on the NC membrane of the cassette. Extraction vial: 0.5mL per vial containing borate buffer and surfactant for sample extraction. Sterile disposable swab: 0197 MDD 93/42/EEC.
Technical data (sensitivity values)
Sensitivity: 95.4% | Specificity: 99.6%
• For nasopharyngeal swab, the sensitivity was 91.91% (125/136) (95% CI 86.10%-95.42%) and the specificity was 98.55% (339/344) (95% CI 96 .64%-99.38%).
• For anterior nasal swab, the sensitivity was 89.76% (228/254) (95% CI 85.42%-92.92%) and the specificity was 99.61% (506/508) (95% CI 98.58% -99.89%).
• For saliva samples, the sensitivity was 89.04% (130/146) (95% CI 82.94%-93.14%) and the specificity was 99.40% (334/336) (95% CI 97.86%- 99.84%).
Instructions for use (sampling)
Anterior nasal swab:
1. Take the swab out of the container being careful not to touch the soft end which is the absorbent tip.
2. Insert the entire absorbent tip of the swab into your nostril.
3. Slowly rotate the swab in a circular motion against the inside of your nostril at least 4 times for a total of 15 seconds. Be sure to collect any nasal drainage that may be present on the swab.
4. Carefully remove the swab.
5. Repeat steps 2-4 with the same swab in the other nostril.
Saliva Sample:
1. Take the swab out of the container being careful not to touch the soft end which is the absorbent tip.
2. Insert the entire absorbent tip of the swab into your mouth.
3. Use the swab to slowly wipe the upper palate and the inside of the left and right cheeks. Be sure to collect saliva present on the swab.
4. Carefully remove the swab.
Evaluation of the test results
Invalid test run: If no red line appears next to the control zone (C), the test is invalid. Discard the test cassette and repeat with a new sample and a new cassette.
Positive results: A red line appears next to the test zone (T). Another line appears next to the control zone (C), indicating that SARS-CoV-2 nucleocapsid antigen was detected by this test.
Negative results: No red line appears next to the test zone (T) and a line appears next to the control zone (C), indicating that no SARS-CoV-2 nucleocapsid antigen was detected with this test . However, this does not exclude the possibility of infection with SARS-CoV-2.
The positive result obtained with the WANTAI SARS-CoV-2 Ag Rapid Test (Colloidal Gold) alone cannot be the definitive diagnosis of COVID-19. Individuals who test positive with the WANTAI SARS-CoV-2 Ag Rapid Test (Colloidal Gold) should self-isolate and seek follow-up from their doctor or healthcare provider, as additional testing may be required.
Negative results should be considered presumptive, do not preclude SARS-CoV-2 infection, and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of an individual’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID19 and, where appropriate, confirmed with a molecular assay for patient management. Individuals who test negative and continue to have COVID-like symptoms of fever, cough, and/or shortness of breath may still have SARS-CoV-2 infection and should seek follow-up care from their doctor or healthcare provider.
Downloads (product certificates)
Manufacturer: Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.